Vanda Pharmaceuticals Inc. VNDA revealed that the FDA has sent a complete response letter denying approval for its new drug application (NDA) for tradipitant, a potential treatment for gastroparesis symptoms.
In response to the news, the company’s shares plummeted by 6.1% on September 19.
Gastroparesis, characterized by delayed gastric emptying, impedes the stomach’s ability to process its contents swiftly. Notably, the FDA has not endorsed any new medications for gastroparesis treatment in over four decades.
Since the beginning of the year, Vanda’s stock has risen by 10.2%, outperforming the industry’s overall growth of 0.4%.
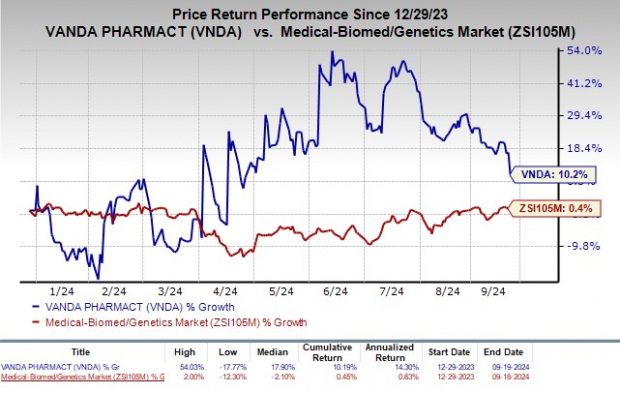
Image Source: Zacks Investment Research
Analysis of FDA’s Complete Response Letter on Tradipitant
According to the complete response letter, the FDA has requested Vanda to conduct additional studies on tradipitant using a methodology that diverges from recommendations by leading experts and lacks scientific merit in addressing the disease.
Additionally, the company stated that the FDA’s decision was delayed by over 185 days and failed to adhere to the stipulations of the Food Drug and Cosmetic Act (FDCA).
Under the FDCA, the FDA is obligated to review an NDA and issue either an approval or a hearing invitation within 180 days post-submission. The FDA did not fulfill this requirement.
Vanda reported that it persistently urged the FDA to organize an advisory committee meeting to discuss the tradipitant NDA, which the regulatory body repeatedly rejected.
Subsequently, several patients treated with tradipitant have submitted a Citizen Petition imploring the FDA to approve tradipitant for gastroparesis treatment.
Vanda’s Future Plans for Tradipitant Development
Despite the FDA’s rejection for gastroparesis, Vanda intends to pursue marketing authorization for tradipitant in this specific indication. Additionally, the company plans to explore tradipitant for preventing motion sickness-induced vomiting.
In May 2024, Vanda released positive results from a second phase III trial examining tradipitant’s efficacy in preventing vomiting induced by motion sickness.
The company aims to submit an NDA for tradipitant with the FDA for motion sickness treatment later in 2024.
Stock Analysis and Recommendations
Currently, Vanda holds a Zacks Rank #4 (Sell).
Other well-performing biotech stocks include Illumina, Inc. ILMN, Krystal Biotech, Inc. KRYS, and Fulcrum Therapeutics, Inc. FULC, each of which currently holds a Zacks Rank #1 (Strong Buy).
In the last 60 days, projected earnings per share for Illumina in 2024 have surged from $1.84 to $3.63, with 2025 estimates climbing from $3.22 to $4.43. Nonetheless, year to date, ILMN shares have dipped by 3.5%.
Illumina has consistently exceeded earnings projections in the past four quarters, with an average surprise of 463.46%.
Over the last 60 days, estimated earnings per share for Krystal Biotech in 2024 have increased from $2.09 to $2.38, and from $4.33 to $7.31 for 2025. KRYS shares have witnessed a rise of 48.7% year to date.
Krystal Biotech surpassed earnings expectations in three of the last four quarters, with an average surprise of 45.95%.
During the last 60 days, projected loss per share for Fulcrum Therapeutics in 2024 has narrowed from $1.33 to 28 cents, and from $1.71 to $1.14 for 2025. FULC shares have experienced a 48.9% decline year to date.
Fulcrum Therapeutics surpassed earnings estimates in each of the previous four quarters, with an average surprise of 393.18%.
